Beyond serialization: building protection against falsified medicines
Around the world, regulators have taken strong action against falsified medicines. Serialization, aggregation, traceability requirements, and tamper-evident systems are now part of the pharmaceutical industry’s compliance landscape.

Beyond serialization
- In the United States, the Drug Supply Chain Security Act (DSCSA) requires product serialization and full traceability across the supply chain.
- In the European Union, the Falsified Medicines Directive (FMD) mandates unique identifiers and tamper-evident features on prescription medicines.
- In countries like Russia, Brazil, India, and China, national track-and-trace programs impose strict serialization and aggregation requirements.
These are important steps—but they are not sufficient.
Serialization: A first step, but not enough
Serialization was designed as a regulatory safeguard, not as a comprehensive security solution. While unique codes on medicine packages create traceability, they also expose new vulnerabilities:
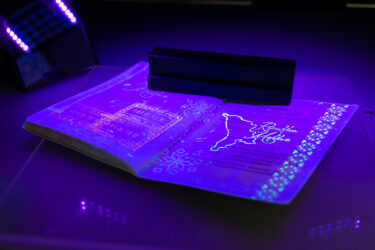
- Codes can be stolen. A legitimate code can be removed and reapplied to a counterfeit product.
- Codes can be cloned. Anyone can read a visible code with a mobile app and replicate it on fake packaging.
- Detection is too late. The system only raises an alert when a duplicate serial number is detected. If the counterfeit reaches the shelf first, there is no way of knowing it was fake, leaving patients exposed.
In other words, serialization ensures regulatory compliance and supply chain traceability, but it does not guarantee full protection.
The next frontier: secure protection
To truly protect patients and safeguard brands, the industry must go beyond compliance. At Edgyn, we believe serialization is just the beginning. To truly safeguard patients, pharmaceutical companies must adopt advanced, layered solutions that go beyond visible codes.
That’s why we developed Edgyn’s Digital Fingerprint technology—a breakthrough that takes supply chain security to the next level. By analyzing the microscopic variations of each package’s material, Digital Fingerprint creates a unique digital identity for every product—just like a human fingerprint.
- Impossible to replicate: Every product has a unique, inherent identity that counterfeiters cannot copy.
- Non-intrusive: No changes to manufacturing or packaging are required.
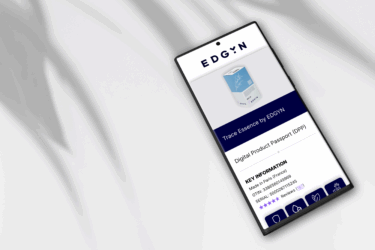
- Instant smartphone authentication: Inspectors, distributors, and even consumers can verify authenticity within seconds.
- Beyond authentication: The solution can also detect product diversion, signaling when a genuine item appears in a market where it was not intended to be sold.
Safeguarding Patients, Protecting Trust
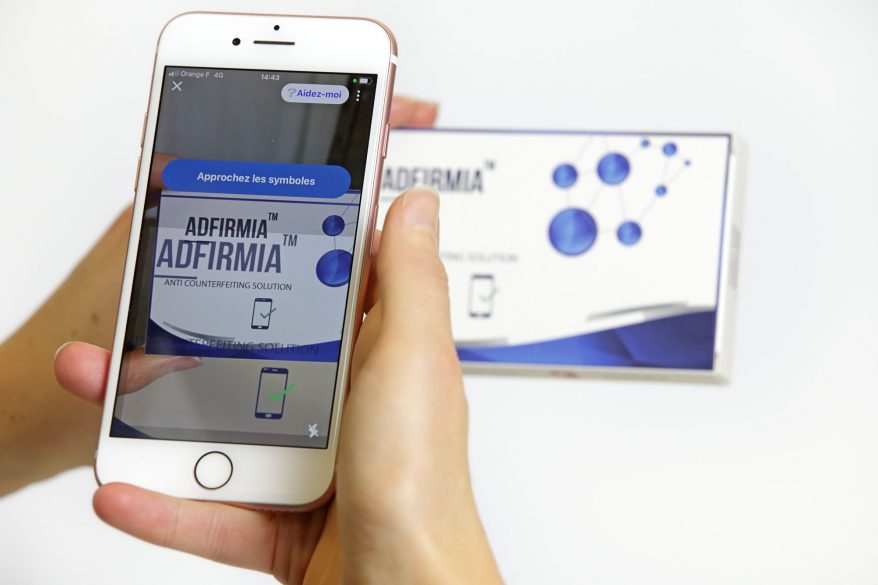
Serialization has raised the bar for compliance and traceability. But as counterfeiters evolve, so must our defenses. To truly protect patients and safeguard the trust that underpins every pharmaceutical brand, companies must embrace advanced, layered security.
With Edgyn’s Digital Fingerprint technology, the industry can close the gaps left by serialization, prevent falsified medicines from reaching patients, and build a more secure and trustworthy supply chain.
Take the Next Step in Medicine Protection
With Edgyn’s Digital Fingerprint, you can secure your supply chain, protect patients, and strengthen brand trust.

Discover more
 Pharma Case Study
Pharma Case Study Industry
IndustryPharma